Understanding Atomic Structure: The Building Blocks of Matter
Ever wondered what makes up everything around you? It all begins with atoms! These tiny particles, the fundamental units of matter, are composed of three main subatomic particles: protons, neutrons, and electrons. Think of an atom as a miniature solar system: positively charged protons (+) reside in the atom's core, the nucleus, along with neutral neutrons (0). Negatively charged electrons (-) zoom around the nucleus in various energy levels or shells.
Defining Key Terms
Let's define some crucial terms to help you navigate the atomic world:
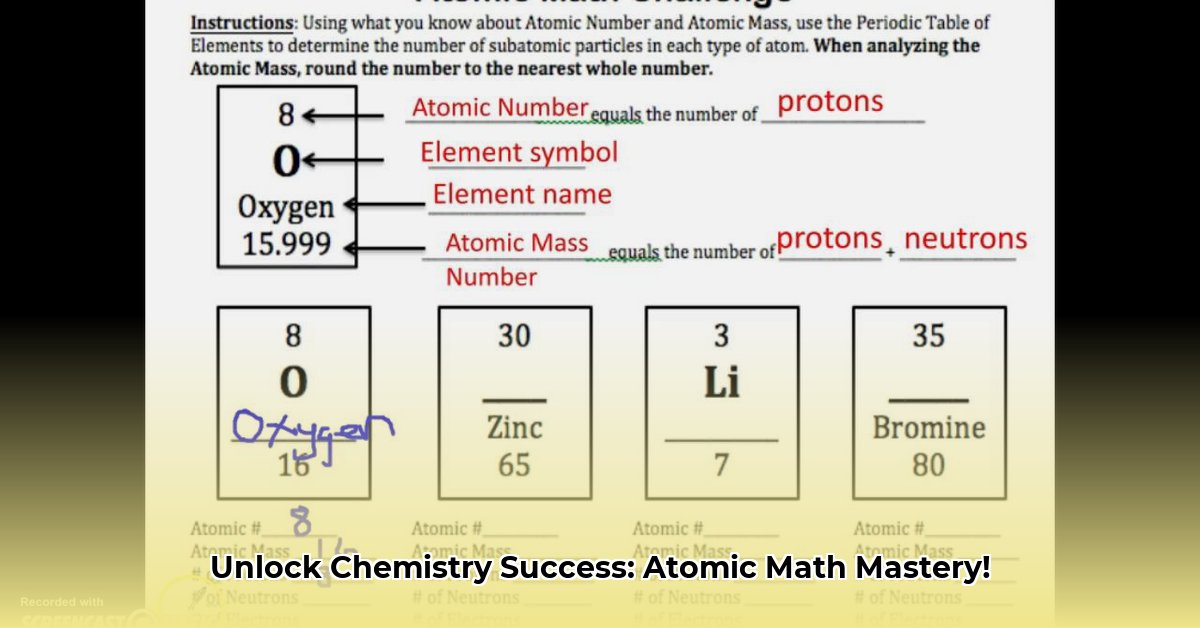
- Atomic Number: The number of protons in an atom's nucleus. This number uniquely identifies an element (e.g., hydrogen has an atomic number of 1, helium 2, etc.).
- Atomic Mass (Mass Number): The total number of protons and neutrons in an atom's nucleus. This is approximately the atom's mass in atomic mass units (amu). Electrons are so light, their mass is negligible in atomic mass calculations.
- Isotopes: Atoms of the same element having the same atomic number but different numbers of neutrons. This means they have the same number of protons but different atomic masses. For example, Carbon-12 and Carbon-14 are isotopes of carbon. Both have 6 protons, but Carbon-12 has 6 neutrons, while Carbon-14 has 8.
- Weighted Average Atomic Mass: The average atomic mass of an element considering the abundance of its isotopes. The value you see on the periodic table is a weighted average, reflecting the relative proportions of each isotope in a naturally occurring sample.
Calculating Neutrons: A Simple Subtraction
Determining the number of neutrons in an atom is straightforward: subtract the atomic number (number of protons) from the atomic mass (total number of protons and neutrons).
Example: An atom of oxygen has an atomic number of 8 and an atomic mass of 16. Therefore, it has 16 - 8 = 8 neutrons. Isn't that easy?
Isotopes and Their Impact on Atomic Mass
Did you know that most elements exist as a mixture of isotopes? The relative abundance of each isotope influences the element's weighted average atomic mass, the value you'll find on the periodic table. This average reflects the natural proportion of isotopes found in a typical sample of the element.
Calculating Weighted Average Atomic Mass: A Step-by-Step Guide
Calculating the weighted average atomic mass requires considering the mass and relative abundance of each isotope present.
- Identify Isotopes and Masses: Determine all the naturally occurring isotopes of the element and their respective masses (in amu).
- Determine Isotopic Abundances: Find the percentage abundance of each isotope. This information is typically provided in the problem.
- Convert Percentages to Decimal Fractions: Divide each percentage abundance by 100 to obtain the decimal fraction representing the fractional abundance of each isotope.
- Calculate Weighted Average: For each isotope, multiply its mass (amu) by its fractional abundance. Then, sum the results to obtain the weighted average atomic mass.
Example: Chlorine has two main isotopes: ³⁵Cl (34.97 amu, 75.77% abundance) and ³⁷Cl (36.97 amu, 24.23% abundance).
Weighted average atomic mass of Chlorine = (34.97 amu * 0.7577) + (36.97 amu * 0.2423) = 35.45 amu
This closely matches the value shown on the periodic table!
Practice Problems: Sharpen Your Atomic Skills
Test your understanding with these practice problems:
| Element | Atomic Number | Atomic Mass | Number of Neutrons |
|---|---|---|---|
| Carbon (¹²C) | 6 | 12 | |
| Oxygen (¹⁶O) | 8 | 16 | |
| Sodium (²³Na) | 11 | 23 |
(Answers provided at the end of the article to avoid influencing your calculations.)
Real-World Applications
Understanding atomic structure and isotopic variations isn't just for textbooks. It has critical applications in diverse fields:
- Nuclear Medicine: Radioisotopes (isotopes with unstable nuclei) are used in medical imaging and treatments.
- Geochronology: Isotope ratios help scientists date geological formations and artifacts.
- Environmental Science: Isotopic analysis aids in tracking pollutant sources and monitoring environmental changes.
Key Takeaways
- Atoms consist of protons, neutrons, and electrons.
- Atomic number reflects the number of protons, defining an element.
- Atomic mass represents the total number of protons and neutrons.
- Isotopes are variants of an element with the same atomic number but different atomic masses.
- The weighted average atomic mass considers the abundance of each isotope.
Mastering these fundamental concepts unlocks a deeper appreciation for the amazing world of chemistry and its impact on our lives!
(Answers to practice problems: Carbon: 6 neutrons; Oxygen: 8 neutrons; Sodium: 12 neutrons.)